- Product Details
Keywords
- low price Ethyl difluoroacetate
- top quality Ethyl difluoroacetate
- Ethyl difluoroacetate China supplier
Quick Details
- ProName: Ethyl difluoroacetate
- CasNo: 454-31-9
- Molecular Formula: C4H6F2O2
- Appearance: colorless transparent liquid
- Application: Ethyl difluoroacetate could react with...
- PackAge: 50kg/drum
- ProductionCapacity: Metric Ton/Day
- Purity: 98%
- Storage: store in a cool,dry. Well ventilated p...
- Transportation: flammable liquid, class: 3; UN1992, p...
- LimitNum: 0 Metric Ton
Superiority
we have a land of 3 hectares and an experienced R&D team.
We establish closely cooperative relationships with Zhejiang University, Zhejiang University of Science and
Technology, Emden-Leer of Germany, etc.
Company Name: Nantong Baokai Chemical Co., Ltd
Year Established: 2006
Main products: fluoro-pharmaceutical intermediates
Details
1. Introduction of Ethyl difluoroacetate (CAS No. 454-31-9)
Our Ethyl difluoroacetate products are mainly Ethyl difluoroacetate 98%. As one kind of clear colorless liquid, it is stored in a cool, dry, well ventilated place. The package of this chemical is 50kg/drum, and its transportation information are flammable liquid, class: 3; UN1992.
Our Certificate Of Analysis:
| Test Item | Sandard | Test Result |
| Batch No. | - | 20120516 |
| Apprearance | Colorless Liquid | Colorless Liquid |
| Purity |
≥99.5% |
99.71% |
| Water | ≤0.1% | 0.07% |
| Weight | - | 10 |
For being stable in normal condition, Ethyl difluoroacetate is incompatible with strong oxidizing agents. And the hazardous decomposition products are including carbon monoxide, carbon dioxide, hydrogen fluoride gas. When comes to Ethyl difluoroacetate solubility, it is slight soluble in water, while miscible with organic materials like alcohol and ether.
Ethyl difluoroacetate should be stored sealed in the container in a cool, dry place, and it should be kept away from sources of ignition.
2. Properties of Ethyl difluoroacetate (CAS No. 454-31-9)
The properties of Ethyl difluoroacetate are as below: (1)ACD/LogP: 0.661; (2)ACD/LogD (pH 5.5): 0.66; (3)ACD/LogD (pH 7.4): 0.66; (4)ACD/BCF (pH 5.5): 1.87; (5)ACD/BCF (pH 7.4): 1.87; (6)ACD/KOC (pH 5.5): 54.50; (7)ACD/KOC (pH 7.4): 54.50; (8)#H bond acceptors: 2; (9)#Freely Rotating Bonds: 3; (10)Polar Surface Area: 26.3; (11)Index of Refraction: 1.336; (12)Molar Refractivity: 22.641 cm3; (13)Molar Volume: 109.317 cm3; (14)Polarizability: 8.976×10-24cm3; (15)Surface Tension: 20.4349994659424 dyne/cm; (16)Density: 1.135 g/cm3; (17)Flash Point: 1.121 °C; (18)Enthalpy of Vaporization: 31.712 kJ/mol; (19)Boiling Point: 76.028 °C at 760 mmHg; (20)Vapour Pressure: 102.511001586914 mmHg at 25°C.
3. Structure Descriptors of Ethyl difluoroacetate (CAS No. 454-31-9)
You could convert the following datas into the molecular structure:
(1). InChI:InChI=1S/C4H6F2O2/c1-2-8-4(7)3(5)6/h3H,2H2,1H3
(2). InChIKey:InChIKey=GZKHDVAKKLTJPO-UHFFFAOYSA-N
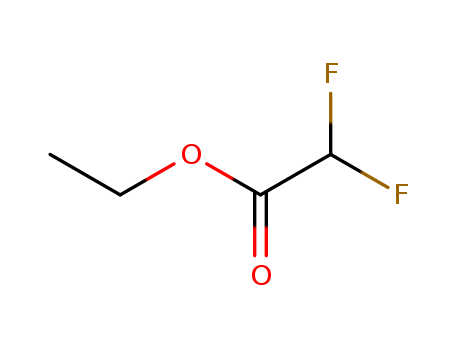
(3). Smiles:C(OCC)(C(F)F)=O
4. Safety Information of Ethyl difluoroacetate (CAS No. 454-31-9)
(1). Irritant. For being irritating to eyes and respiratory system, Ethyl difluoroacetate may cause inflammation to the skin or other mucous membranes, and it may toxic by inhalation.
(2). Corrosive: This chemcial may destroy living tissue on contact. And it is irritating to respiratory system.
(3). Toxic. Ethyl difluoroacetate could at low levels cause damage to health.
(4). Highly flammable: This chemcial may catch fire in contact with air, only needing brief contact with an ignition source, and it has a very low flash point or evolve highly flammable gases in contact with water. And it could causes burns.
Therefore, you should take the following measure to prevent from danger. Wear suitable protective clothing, gloves and eye/face protection, and take off immediately all contaminated clothing when contact. If in case of contact with eyes, rinse immediately with plenty of water and seek medical advice; If in case of accident or if you feel unwell seek medical advice immediately (show the label where possible). Besides, keep away from sources of ignition - No smoking.
5. Use of Ethyl difluoroacetate (CAS No. 454-31-9)
Ethyl difluoroacetate could react with phenethylmagnesium bromide to produce 1,1-difluoro-4-phenyl-butan-2-one. This reaction could happen with the solvent of diethyl ether, with the reaction time of 4 hour(s) and reaction temperature of -70 °C. And its yield is 84 %.